The pentose phosphate pathway (PPP), also known as the direct oxidative pathway, glycolytic shunt, hexose monophosphate shunt (HMS), or Warburg-Lipmann-Dickens cycle, is an alternative route for glucose breakdown that bypasses glycolysis and the Krebs cycle. This pathway, present in both prokaryotes and eukaryotes, occurs in the cytoplasm and chloroplasts under aerobic conditions. Unlike other respiratory pathways, the PPP generates NADPH instead of NADH. The primary substrate for this pathway is glucose-6-phosphate.
Introduction to the Pentose Phosphate Pathway
In contrast to the conventional glucose breakdown involving glycolysis, the Krebs cycle, and the electron transport system (ETS), the pentose phosphate pathway (PPP) offers an alternative route. This pathway is operational in both prokaryotic and eukaryotic cells, taking place in the cytoplasm and chloroplasts in the presence of oxygen. A key distinction is that the PPP produces NADPH instead of NADH, making it unique among respiratory pathways. The starting molecule for this pathway is glucose-6-phosphate.
Stoichiometry and Intermediates
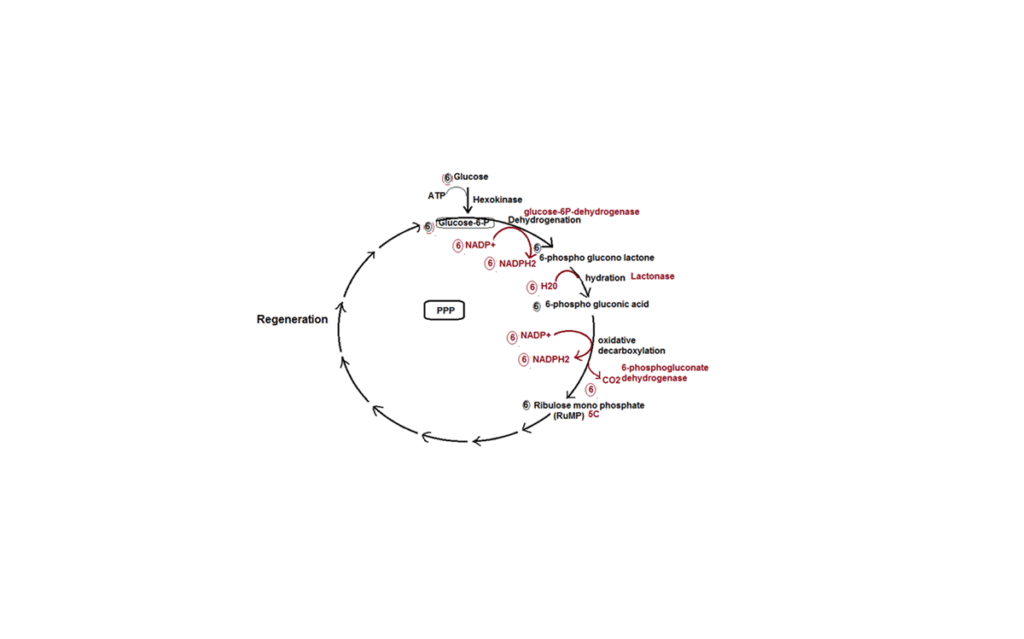
In the PPP, six molecules of glucose-6-phosphate enter the pathway, but only five are regenerated. The loss of one molecule is due to the release of six molecules of CO2. During the regeneration of glucose-6-phosphate from ribulose-5-phosphate (RuMP), several intermediate compounds are formed, including erythrose-4-phosphate (4C), xylulose-5-phosphate (5C), ribulose-5-phosphate (5C), fructose-6-phosphate (6C), and sedoheptulose-7-phosphate (7C).
Significance of NADPH Production
The PPP generates 12 NADPH2 molecules, equivalent to 36 ATP molecules if they were to be used in oxidative phosphorylation. However, the primary purpose of the PPP is not ATP synthesis. Instead, it focuses on producing reducing power in the form of NADPH2 and generating intermediate compounds crucial for synthesizing various essential molecules within the cell. This dual role in both breakdown and synthesis classifies the PPP as an amphibolic pathway.
Significance of the Pentose Phosphate Pathway
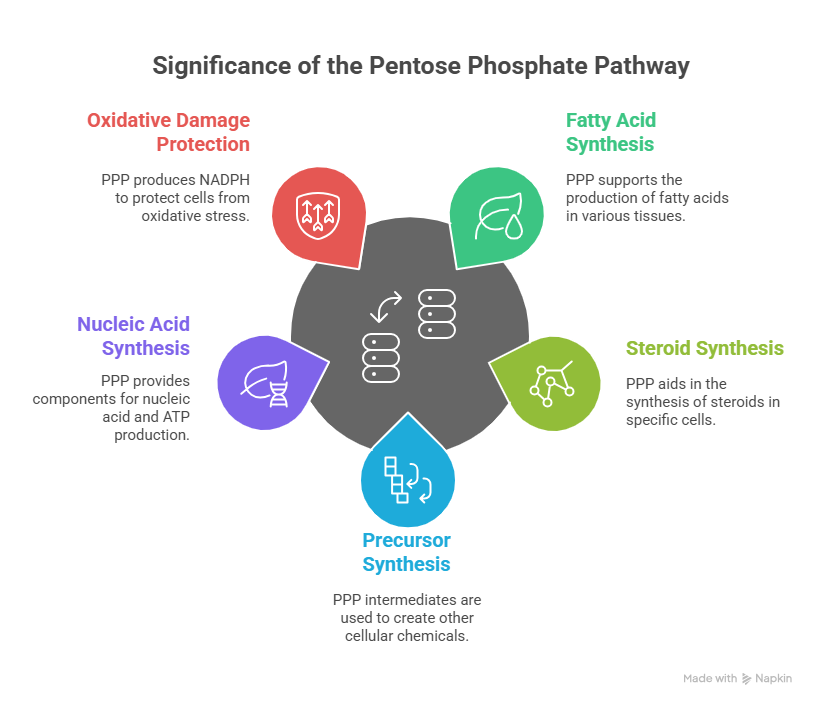
The pentose phosphate pathway holds several key significances:
-
Fatty Acid and Steroid Synthesis: The PPP is highly active in cells involved in fatty acid and steroid synthesis, such as those found in mammary glands, testes, ovaries, adipose cells, fatty seeds, and the liver.
-
Precursor Synthesis: Intermediates produced in the PPP serve as precursors for synthesizing various chemicals within the cell. For example, erythrose-4-phosphate is used in the formation of lignin, auxin, phenolic compounds, and phytoalexins.
-
Nucleic Acid Synthesis: Ribulose-5-phosphate is utilized for the synthesis of nucleic acids and ATP.
-
Protection Against Oxidative Damage: The PPP plays a crucial role in protecting cells from oxidative damage by producing NADPH, which is essential for reducing oxidative stress.
Glucose-6-Phosphate Dehydrogenase Deficiency
In some individuals, a genetic condition known as glucose-6-phosphate dehydrogenase (G6PD) deficiency, an X-linked recessive disorder, results in the absence of the G6PD enzyme. This deficiency leads to decreased NADPH production, reducing the breakdown of H2O2 and increasing its toxicity, ultimately causing oxidative damage to cells.
Patients with G6PD deficiency should avoid certain substances, such as anti-malarial drugs like primaquine and sulfa drugs, as well as fava beans. These compounds can further increase H2O2 production, exacerbating the existing oxidative damage.
Resistance to Malaria
Interestingly, G6PD deficiency offers a protective benefit against malaria. The enzyme deficiency increases oxidative damage to red blood cells (RBCs), which are particularly sensitive to oxidative stress. The malaria parasite Plasmodium falciparum is also highly susceptible to oxidative damage. Consequently, individuals with G6PD deficiency exhibit increased resistance to malaria infection.
FAQs (Frequently Asked Questions)
1. What is the Pentose Phosphate Pathway (PPP)?
The Pentose Phosphate Pathway (also called the hexose monophosphate shunt or phosphogluconate pathway) is a metabolic pathway parallel to glycolysis. It generates NADPH and pentoses (5-carbon sugars), particularly ribose-5-phosphate, a precursor for nucleotide biosynthesis. The PPP does not directly produce or consume ATP.
2. What are the two main phases of the PPP?
The Pentose Phosphate Pathway consists of two main phases:
-
Oxidative Phase: This irreversible phase generates NADPH and ribulose-5-phosphate.
-
Non-Oxidative Phase: This reversible phase interconverts sugars, allowing the production of ribose-5-phosphate or the channeling of excess sugars back into glycolysis.
3. What is the primary function of the oxidative phase?
The primary function of the oxidative phase is to produce NADPH. This is achieved through two key reactions catalyzed by:
-
Glucose-6-phosphate dehydrogenase (G6PD): Oxidizes glucose-6-phosphate to 6-phosphoglucono-δ-lactone, producing NADPH.
-
6-Phosphogluconate dehydrogenase: Oxidizes 6-phosphogluconate to ribulose-5-phosphate, producing another molecule of NADPH and releasing CO2.
4. What is the role of NADPH produced in the Pentose Phosphate Pathway?
NADPH has several crucial roles:
-
Reductive Biosynthesis: It is a reducing agent used in anabolic reactions, such as fatty acid and steroid synthesis.
-
Antioxidant Defense: It is essential for reducing oxidized glutathione, which is required by glutathione peroxidase to detoxify harmful reactive oxygen species (ROS). This is particularly important in red blood cells.
-
Immune Function: NADPH is used by phagocytes (e.g., neutrophils, macrophages) in the respiratory burst to generate superoxide radicals, which kill bacteria.
5. What is the primary function of the non-oxidative phase?
The primary function of the non-oxidative phase is to interconvert sugars. This allows the cell to:
-
Produce Ribose-5-Phosphate: If the cell needs more ribose-5-phosphate for nucleotide synthesis than NADPH, the non-oxidative phase can convert glycolytic intermediates (fructose-6-phosphate and glyceraldehyde-3-phosphate) into ribose-5-phosphate.
-
Channel Excess Sugars into Glycolysis: If the cell needs more NADPH than ribose-5-phosphate, the ribulose-5-phosphate produced in the oxidative phase can be converted back into glycolytic intermediates (fructose-6-phosphate and glyceraldehyde-3-phosphate).
6. What are the key enzymes in the non-oxidative phase?
The key enzymes in the non-oxidative phase are:
-
Transketolase: Transfers a two-carbon unit. Requires thiamine pyrophosphate (TPP) as a cofactor.
-
Transaldolase: Transfers a three-carbon unit.
7. How is the PPP regulated?
The PPP is primarily regulated at the first committed step, catalyzed by glucose-6-phosphate dehydrogenase (G6PD).
-
NADPH Inhibition: High levels of NADPH inhibit G6PD, slowing down the pathway. This is a form of feedback inhibition.
-
Insulin Stimulation: Insulin stimulates the expression of G6PD, increasing the pathway’s activity. This is particularly important in tissues involved in fatty acid synthesis, such as the liver and adipose tissue.
8. What tissues have high PPP activity?
Tissues with high PPP activity include:
-
Liver: For fatty acid synthesis.
-
Adipose Tissue: For fatty acid synthesis.
-
Adrenal Cortex: For steroid hormone synthesis.
-
Red Blood Cells: To maintain NADPH levels for antioxidant defense.
-
Mammary Gland (during lactation): For fatty acid synthesis.
9. What is Glucose-6-Phosphate Dehydrogenase (G6PD) deficiency?
G6PD deficiency is a common genetic disorder that affects the enzyme glucose-6-phosphate dehydrogenase. It is most prevalent in populations of African, Mediterranean, and Asian descent.
10. What are the consequences of G6PD deficiency?
G6PD deficiency reduces the ability of red blood cells to produce NADPH. This makes them more susceptible to oxidative damage, leading to:
-
Hemolytic Anemia: Red blood cells are prematurely destroyed, leading to anemia. This can be triggered by:
-
Infections: Inflammatory responses generate ROS.
-
Certain Drugs: Some drugs (e.g., antimalarials, sulfonamides) can induce oxidative stress.
-
Fava Beans: Contain vicine and convicine, which are oxidants.
-
-
Neonatal Jaundice: Increased bilirubin levels due to red blood cell breakdown.
11. How is G6PD deficiency diagnosed?
G6PD deficiency is diagnosed by measuring G6PD enzyme activity in red blood cells. Genetic testing can also be used to identify specific mutations in the G6PD gene.
12. How is G6PD deficiency treated?
Treatment for G6PD deficiency focuses on avoiding triggers that can cause hemolytic anemia. This includes:
-
Avoiding certain drugs: Healthcare providers should be informed about the deficiency before prescribing medications.
-
Avoiding fava beans: Dietary restrictions may be necessary.
-
Prompt treatment of infections: Infections can trigger hemolytic episodes.
-
Blood transfusions: May be necessary in severe cases of hemolytic anemia.
13. What is the connection between the Pentose Phosphate Pathway and cancer?
Cancer cells often have increased PPP activity. This is because they require:
-
NADPH: For rapid cell growth and division, including lipid synthesis and maintaining redox balance.
-
Ribose-5-Phosphate: For nucleotide synthesis, which is essential for DNA replication.
Therefore, the PPP is an attractive target for cancer therapy. Inhibiting key enzymes in the PPP could potentially slow down cancer cell growth.
14. Can the Pentose Phosphate Pathway operate in reverse?
While the oxidative phase is irreversible, the non-oxidative phase is reversible. This allows the cell to adjust the flux of the pathway based on its needs for NADPH and ribose-5-phosphate.
15. What is the role of thiamine pyrophosphate (TPP) in the PPP?
Thiamine pyrophosphate (TPP) is a cofactor required by transketolase, a key enzyme in the non-oxidative phase of the PPP. TPP is derived from thiamine (vitamin B1). A deficiency in thiamine can impair transketolase activity and disrupt the PPP.

