In this article, I’ll be discussing the ozone depletion in the stratosphere. This article will include the bad ozone and good ozone, the disruption of ozone formation and degradation by CFCs, the harmful effects of UV rays on humans, and the international treaty to control the emission of ozone-depleting substances.
Factors Responsible for Ozone Depletion
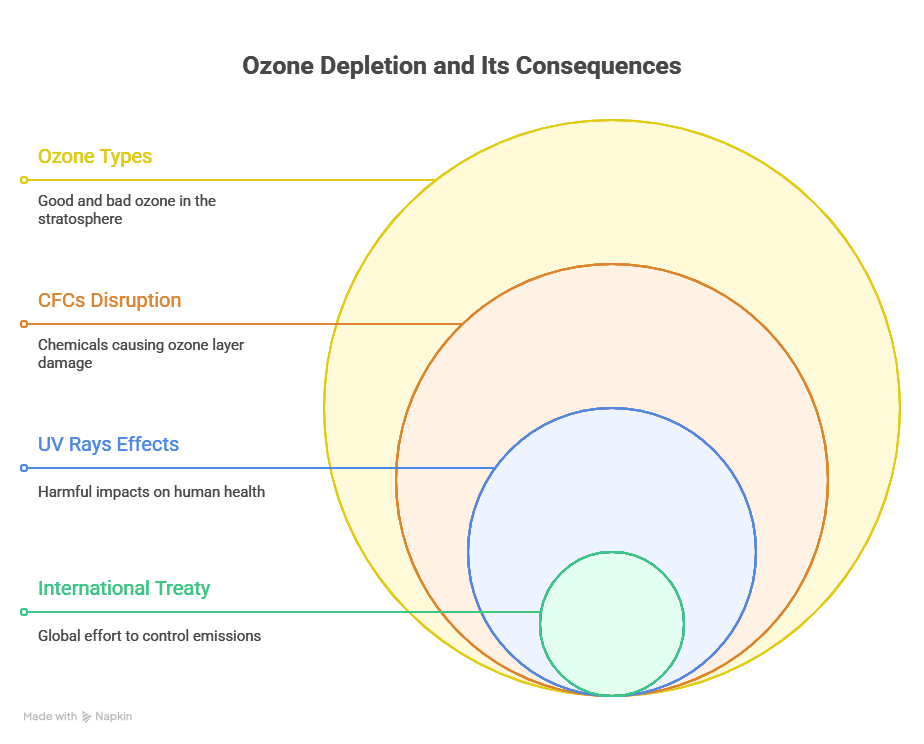
Bad ozone: Unlike good ozone, which is present in the stratosphere, bad ozone also known as chemical weed is present in the troposphere. When we say, weed, it is an unwanted plant, likewise bad ozone is unwanted in the troposphere. Bad ozone formation in the troposphere occurs due to the formation of photochemical smog. In photochemical smog, fossil fuel burning produces hydrocarbons (HC) and NO (nitric oxide). When these pollutants are high in the atmosphere, a chain reaction occurs in the presence of the sunlight.
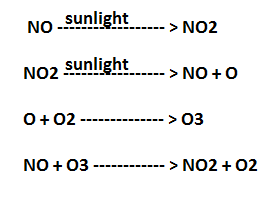
NO2 is a brown gas and at sufficiently high level, it can contribute to haze. O3 is a toxic gas and both NO2 and O3 are strong oxidizing agents which can react with unburnt HC in the polluted air to produce chemicals such as acrolein, formaldehyde (HCHO), and PAN (Peroxyacetyl Nitrate). Both O3 and PAN are serious eye irritants. O3 and NO irritate nose, and throat. At high concentration causes headache, dryness of throat, chest pain, cough, and difficulty breathing.
Good ozone of the Stratosphere
Good ozone is present in the stratosphere at 20-26 km above the sea level. The concentration of good ozone is 0.3 to 1ppm. On the other hand, the concentration of bad ozone in the troposphere is 0.05 ppm. Good ozone acts as a shield absorbing UV radiation from the Sun. Otherwise, UV radiation is highly injurious. DNA and proteins of living organisms preferentially absorb UV rays, but the high energy content of the UV rays break the chemical bonds within these molecules. This results in severe diseases in humans including cancer. Dobson unit (DU) is used for measuring the thickness of the ozone layer.
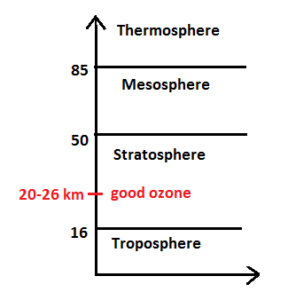
I DU is equal to the number of ozone molecules needed to create a pure layer of ozone 0.01 mm or 10 micrometer thickness at STP. For example, 300 DU means 3 mm layer of ozone gas at STP.
Formation of ozone:
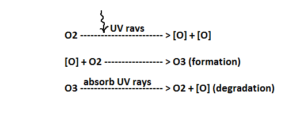
There is a balance between formation and degradation of ozone. A steady layer of ozone is maintained at stratosphere. However, this balance has been disrupted by CFCs (Chloro Fluoro Carbon). CFCs are non-reactive, non-inflammable and non-toxic and that’s why, it gets mixed with the atmospheric gases and eventually reach stratosphere. In stratosphere, CFCs are broken down by UV radiation releasing chlorine free radical.

The continuously generated chlorine ion causes breakdown of ozone and thus the ozone layer. 1 chlorine atom has the ability to dissociate 100,000 ozone molecules. Nowadays, CFCs are being replaced by HFCs (Hydro Fluoro Carbon).
Although ozone depletion is widely occurring in the stratosphere, the severe depletion of ozone (ozone hole) has occurred in Antarctica. This is due to very low temperature in Antarctica leading to the formation of polar stratospheric clouds (PSCs). Special reactions occur in PSCs plus chlorine and bromine reactions are responsible for ozone hole in Antarctica.
Effects of Ozone Depletion: Due to ozone depletion, UV-rays reach the earth surface and cause severe problems. Few harmful effects of UV-rays are mentioned below:
-
- 1% ozone depletion — the load of UV-B rays on the earth surface increases by two times.
-
- UV rays show mutagenic effect.
-
- UV – rays cause cancer
-
- UV-rays can be absorbed by cornea due to which cornea swells and cause snow blindness and cataract.
-
- UV radiation cause formation of thymine dimmers
-
- UV rays are lethal because it causes inactivation of proteins, nucleic acids and pigments.
Types of UV-rays:
Three types of UV-rays are present: UV-A, UV-B and UV-C. UV radiation ranges from 100 – 400 nm.
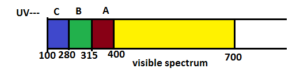
UV-A – near to the visible light ranges from 315 – 400 nm
UV-B – ranges from 280 – 315 nm (Damages DNA and causes mutation). In humans, cornea absorbs UV-B radiation and a high dose of UV-B cause inflammation of cornea, called SNOW-BLINDNESS, cataract, etc. Such exposure may permanently damage the cornea.
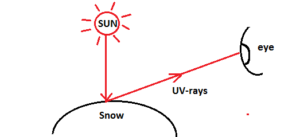
Exposure of UV-B Radiation Cause Snow Blindness
Snow blindness is a short-term symptom when the eye is exposed to UV-light. After some time, the condition gets normal. Common symptoms of snow blindness include tears, eyelid twitching, discomfort from bright light and constricted pupils.
Cause of Snow blindness:
Causal agent – brighter sunlight reflected from snow or ice. Fresh snow reflects about 80% of the UV-radiation.
Prevention: Use of UV-protected glass
UV-C – ranges from 100 – 280 nm
Out of the three UV-rays, UV-C is most lethal, but it does not reach the earth surface as it is prevented by the ozone layer. Only UV-B and UV-A reach the earth surface of which UV-B is most deadly for humans.
International Treaty (Montreal Protocol) was signed at Montreal, Canada in 1987, effective in 1989 to control the emission of ozone depleting substances (CFCs).

