What is Northern Blotting?
Northern blotting technique in molecular biology is used to detect RNA in a cell. We can also say that to analyze the gene expression in a cell – Northern blotting technique is used. What do we mean by gene expression? As we know that a cell contains nucleus and inside the nucleus – DNA is present. When DNA is expressed, RNA formation takes place. In other words, we can say that the expression of genes (present on DNA) results in the synthesis of RNA. Up to what extent a gene expresses and how much RNA is synthesized – this can be identified through Northern blotting. For example, if we talk about a cancer cell – the tumor suppression gene is less expressed compared to oncogenes. So, if we want to study a particular gene in a cancer cell i.e., the expression of that gene in a cancer cell – that can be identified through Northern blotting.
We can take another example where Northern blotting technique is used. For instance, if we are using some drugs for the treatment of cancer cells – we would need to know what changes took place in the expression of genes by the use of such drugs. Whether some gene expressed more or less – these finding can be identified using Northern blotting technique. Now, we will see how this Northern blotting technique is performed.
Northern Blotting Steps:
- RNA is isolated from the cell
- Gel electrophoresis is used to separate RNAs based on their molecular weight. Gel electrophoresis technique is similar to DNA extraction, but for Northern blotting – gel electrophoresis involves one more ingredients, i.e., formaldehyde. Why formaldehyde is used? We know that RNA is a linear structure and RNA in most cases present in its secondary form. So, when we isolate RNA in a gel – we want RNA to be in a linear form. To maintain RNA in its linear form — gel is added with formaldehyde.
- Transfer of RNAs into the nylon / nitrocellulose membrane – for this purpose, a tray is taken which is then filled with transfer buffer solution. A support like sponge is place on the tray. Then the gel is placed on the sponge. This is the same gel that has been used in gel electrophoresis. On the gel, a similar sized nylon / nitrocellulose membrane is kept. On top of the membrane, Whatman gel blot paper is kept followed by paper towels and some weight on top. The buffer solution then starts moving upward due to capillary action. When the buffer solution reaches up to the gel – the RNA present in the gel moves along with the solution towards the nylon membrane. All RNAs are then moved towards the nylon membrane. Once paper towels are wet – it indicates that the transfer process has been completed. Finally, the nylon membrane is removed from the setting.
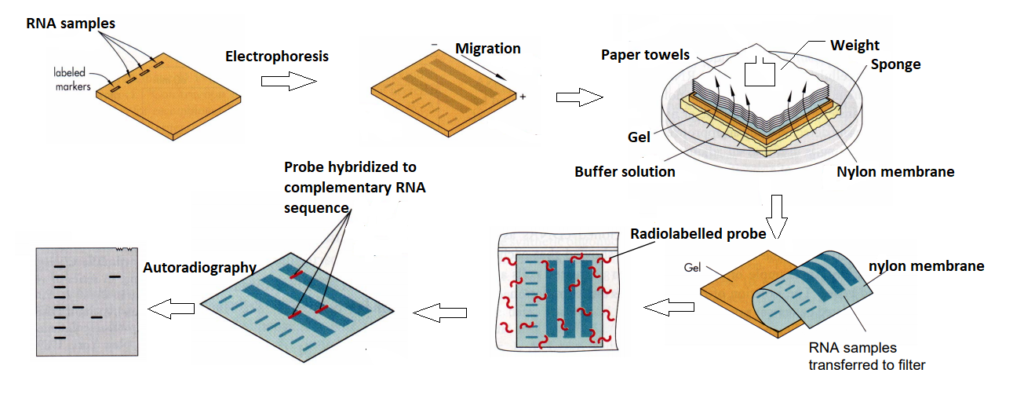
- Fixing of RNAs on the nylon membrane – Once RNAs are transferred to the nylon membrane – they are fixed using UV light or through heat treatment.
Transferring of nylon membrane to a probe solution – for this purpose, a tray is taken and it is filled by a probe solution. After that the nylon membrane is placed in the probe solution. Probes are oligonucleotide sequences (100-150 nucleotides long) attached to a detector molecule at one end. Radiolabelled detector can be used for this purpose. The probe is designed in such a way that it is just the complementary sequence to RNA. When the nylon membrane is placed in the probe solution – the probe present if finds a complementary RNA molecule, it binds to it and hybridizes. If the RNA of our interest is not present on the nylon membrane – no hybridization will occur. This hybridization can be visualized using autoradiography.
Application of Northern Blotting
- To study the expression of genes in tissues, organs
- Diagnosis of several diseases including viral infections
- To study RNA degradation and splicing
- To identify messenger RNA produced by the transgene to protect recombinants
- To study overexpression of oncogenes and down regulation of tumor suppressor gene in myeloma cells

Pingback: Southern Blotting: Technique, Procedure and Applications - Competitors Point